The Effect of Spatial Organization of Chromatin on Morphology of
Eukaryotic Cell Nucleus
-NUCCHROM-


PUBLICATIONS
Cyclic-polymer grafted colloids in spherical confinement: insights for interphase chromosome organization (2023)
Jarosław Paturej & Aykut Erbaş
Physical Biology 2023 https://dx.doi.org/10.1088/1478-3975/ace750
Interphase chromosomes are known to organize non-randomly in the micron-sized eukaryotic cell nucleus and occupy a certain fraction of nuclear volume, often without mixing. Using extensive coarse-grained simulations, we model such chromosome structures as colloidal particles whose surfaces are grafted by cyclic polymers. This model system is known as Rosetta. The cyclic polymers, with varying polymerization degrees, mimic chromatin loops present in interphase chromosomes, while the rigid core models the chromocenter section of the chromosome. Our simulations show that the colloidal chromosome model provides a well-separated particle distribution without specific attraction between the chain monomers. As the polymerization degree of the grafted cyclic chains decreases while maintaining the total chromosomal length (e.g., the more potent activity of condensin-family proteins), the average chromosomal volume becomes smaller, inter-chromosomal contacts decrease, and chromocenters organize in a quasi-crystalline order reminiscent of a glassy state. This order weakens for polymer chains with a characteristic size on the order of the confinement radius. Notably, linear-polymer grafted particles also provide the same chromocenter organization scheme. However, unlike linear chains, cyclic chains result in less contact between the polymer layers of neighboring chromosome particles, demonstrating the effect of DNA breaks in altering genome-wide contacts. Our simulations show that polymer-grafted colloidal systems could help decipher 3D genome architecture along with the fractal globular and loop-extrusion models.
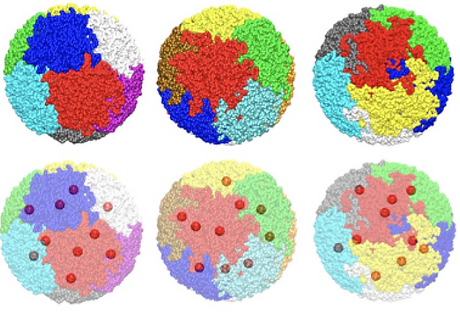
The 3d organization of chromosomes by chromosome territories (shown by different colors) can be facilitated by the cyclic topology of the genome. The bottom row shows the positioning of chromocenters away from each other.
Chromatin phase separation and nuclear shape fluctuations are correlated in a polymer model of the nucleus (2024)
A. Goktug Attar, Jarosław Paturej, Edward J. Banigan, and Aykut Erbaş
Nucleus 2023 https://dx.doi.org/10.1088/1478-3975/ace750
Interphase chromosomes are known to organize non-randomly in the micron-sized eukaryotic cell nucleus and occupy a certain fraction of nuclear volume, often without mixing. Using extensive coarse-grained simulations, we model such chromosome structures as colloidal particles whose surfaces are grafted by cyclic polymers. This model system is known as Rosetta. The cyclic polymers, with varying polymerization degrees, mimic chromatin loops present in interphase chromosomes, while the rigid core models the chromocenter section of the chromosome. Our simulations show that the colloidal chromosome model provides a well-separated particle distribution without specific attraction between the chain monomers. As the polymerization degree of the grafted cyclic chains decreases while maintaining the total chromosomal length (e.g., the more potent activity of condensin-family proteins), the average chromosomal volume becomes smaller, inter-chromosomal contacts decrease, and chromocenters organize in a quasi-crystalline order reminiscent of a glassy state. This order weakens for polymer chains with a characteristic size on the order of the confinement radius. Notably, linear-polymer grafted particles also provide the same chromocenter organization scheme. However, unlike linear chains, cyclic chains result in less contact between the polymer layers of neighboring chromosome particles, demonstrating the effect of DNA breaks in altering genome-wide contacts. Our simulations show that polymer-grafted colloidal systems could help decipher 3D genome architecture along with the fractal globular and loop-extrusion models.
Polymer physics view of peripheral chromatin: de Gennes’ self-similar carpet (2024)
O.S. Sarıyer & Aykut Erbaş
Physical Review E 109, 054403 DOI: https://doi.org/10.1103/PhysRevE.109.054403
Using scaling arguments to model peripheral chromatin localized near the inner surface of the nuclear envelope(NE) as a flexible polymer chain, we discuss the structural properties of the peripheral chromatin composed of alternating lamin-associated domains (LADs) and inter-LADs. Modeling the attraction of LADs to NEby de Gennes’ self-similar carpet, which treats the chromatin layer as a polymer fractal, explains two major experimental observations. (i) The high density of chromatin close to the nuclear periphery decays to a constant density as the distance to the periphery increases. (ii) Due to the decreasing mesh size towards the nuclear periphery, the chromatin carpet inside NE excludes molecules (via nonspecific interactions) above a threshold size that depends on the distance from the nuclear periphery.
Phase behavior and dissociation kinetics of lamins in a polymer model of progeria (2025)
Hadiya Abdul Hameed, Jaroslaw Paturej, and Aykut Erbaş
J. Chem. Phys. 62185101 (2025) DOI: https://doi.org/10.1063/5.0265578
One of the key structural proteins in the eukaryotic cell nucleus is lamin. Lamins can assemble into a two-dimensional protein meshwork at the nuclear periphery, known as the nuclear lamina, which provides rigidity and shape to the nucleus. Mutations in lamin proteins that alter the structure of the nuclear lamina underlie laminopathic diseases, including Hutchinson–Gilford Progeria Syndrome (HGPS). Experiments have shown that, compared to healthy cells, lamin supramolecular structures (e.g., protofilaments) assemble into a thicker lamina in HGPS, where they form highly stable nematic microdomains at the nuclear periphery, reminiscent of liquid crystals. This significantly alters the morphological and mechanical properties of the nucleus. In this study, we investigate the aggregation of lamin fibrous structures and their dissociation kinetics from the nuclear periphery by modeling them as coarse-grained, rod-like polymer chains confined within a rigid spherical shell. Our model reproduces the formation of multidirectional nematic domains at the nuclear surface and the reduced lamin dissociation observed in HGPS nuclei by adjusting lamin concentration, lamin–lamin (head–tail), and lamin–shell association strengths. While nematic phase formation requires relatively strong lamin–shell affinity under any non-vanishing inter-lamin attraction, the thickness of the lamina layer is primarily controlled by the head–tail association strength in the model. Furthermore, the unbinding kinetics of lamin chains from the lamina exhibit a concentration-dependent facilitated dissociation, suppressed by strong intra-lamin interactions, reminiscent of diseased nuclei. Overall, our calculations reveal the physical mechanisms by which mutations affecting native lamin interactions and concentration could lead to an abnormal nuclear lamina in laminopathic diseases.
Peripheral heterochromatin tethering is required for chromatin-based nuclear mechanical response (2025)
A. Goktug Attar, Jarosław Paturej, Ozan S. Sariyer, Edward J. Banigan, and Aykut Erbaş
https://www.biorxiv.org/content/10.1101/2025.02.12.637704v1.abstract (Under review)
The cell nucleus is a mechanically responsive structure that governs how external forces affect chromosomes. Chromatin, particularly transcriptionally inactive heterochromatin, resists nuclear deformations through its mechanical response. However, chromatin also exhibits liquid-like properties, casting ambiguity on the physical mechanisms of chromatin-based nuclear elasticity. To determine how heterochromatin strengthens nuclear mechanical response, we performed polymer physics simulations of a nucleus model validated by micromechanical measurements and chromosome conformation capture data. The attachment of peripheral heterochromatin to the lamina is required to transmit forces directly to the chromatin and elicit its elastic response. Thus, increases in heterochromatin levels increase nuclear rigidity by increasing the linkages between chromatin and the lamina. Crosslinks within heterochromatin, such as HP1α proteins, can also stiffen nuclei, but only if chromatin is peripherally tethered. In contrast, heterochromatin affinity interactions that may drive liquid-liquid phase separation do not contribute to nuclear rigidity. When the nucleus is stretched, gel-like peripheral heterochromatin can bear stresses and deform, while the more fluid-like interior euchromatin is less perturbed. Thus, heterochromatin’s internal structure and stiffness may regulate nuclear mechanics via peripheral attachment to the lamina, while also enabling nuclear mechanosensing of external forces and external measurement of the nucleus’ internal architecture.